Multiple sclerosis (MS) affects more than 2 million people worldwide and is the most common non-traumatic cause of disability primarily amongst American and European adults. It is a multifocal CNS pathology constituting two distinct clinical phases corresponding to inter-related pathological processes: focal inflammation that drives activity during the relapse-remitting stage and neurodegeneration that underlies progressive disease characterized by accumulating fixed disability. Although important advances in treatment to reduce relapse rate have been made in the past two decades, no treatments are available for patients with neurodegenerative MS. There is therefore a significant unmet clinical lacuna for the development of neuroprotective treatments. The development of new therapeutic strategies is necessary to reduce the worldwide social and economic impact of neurodegenerative diseases, which produces high rates of morbidity.
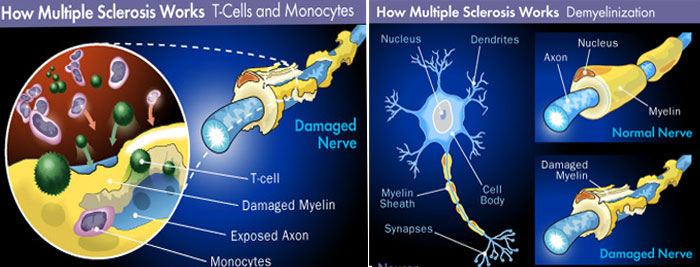
MS causes the removal of fatty myelin sheath from axons of the brain and spinal cord resulting reduced communication among nerve cells. Clinically MS is classified into: Primary progressive MS (PPMS), Relapsing remitting MS (RRMS), Secondary progressive MS (SPMS) and progressive relapsing MS. About 90% of the MS is relapsing-remitting type, which is characterized by periodic attacks (relapses), followed by partial or complete recovery (remissions).It is also common to have a progressive phase of the disease and a large study showed that around 80% of cases followed by chronic progression within 20 years. Primary progressive multiple sclerosis is the prototype neurodegenerative disease. Autoimmunity plays an important role in the disease outcome and body's own immune system attacks on the myelin sheath causing the damage. Several genetic factors including HLA-DR15, HLA-A*02 and HLA-DRB1*1501 are documented to relate with MS. So during inflammatory stage, the immune system gets activated and nerves get demyelinated while during remission stage, immune attack subsides, and the resident oligiodendrocytes remyelinate. There is also evidence of cerebral flow insufficiency into the gray matter in MS suggesting harbinger of neurodegeneration.